
The top plot is for the first hypothesis test (vendor A).Ĭ 1 is the critical value at a significance level of These two separate hypothesis tests are shown graphically below: Therefore, we need to conduct two statistical hypothesis tests. Wrong claim that it is from vendor A should be less than α 2 = 0.05.

It is widely used in quality control in manufacturing and detection ofĪnomalies in medical trials. The test statistic is less than the corresponding upper boundary, so the sequential test does not stop at stage to reject the null hypothesis.The Sequential Probability Ratio Test (SPRT) was developed by Abraham Wald more than a half century ago. The "Test Information" table in Output 81.4.12 displays the boundary values for the test statistic with the specified MLE scale. The PREDPOWER option requests the noninformative predictive power with the observed statistic. The CONDPOWER(CREF=1) option requests the conditional power with the observed statistic under the alternative hypothesis, in addition to the conditional power under the hypothetical reference, the MLE estimate. The data set also provides the boundary information that is needed for the group sequential test at the next stage. The ODS OUTPUT statement with the TEST=TEST_PROP2 option creates an output data set named TEST_PROP2 which contains the updated boundary information for the test at stage. The DATA= option specifies the input data set that contains the test statistic and its associated sample size at stage, and the TESTVAR= option identifies the test variable in the data set.
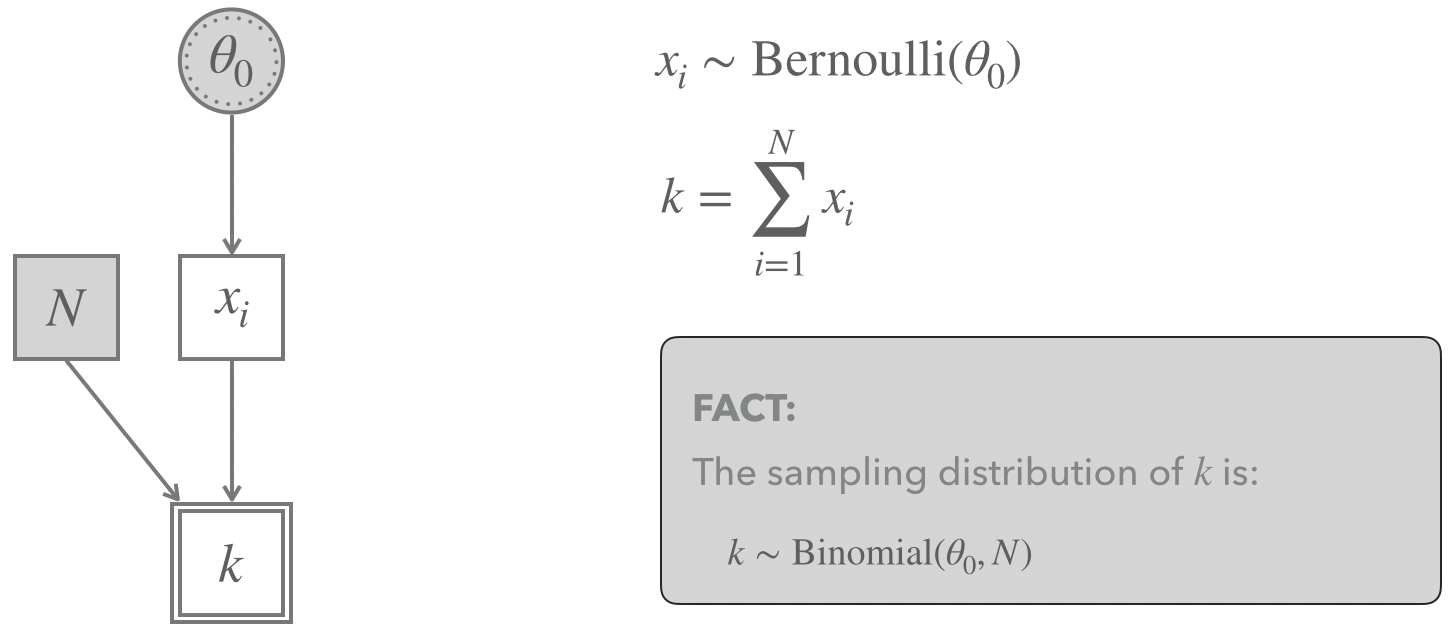
#Binomial sequential testing trial
The BOUNDARY= option specifies the input data set that provides the boundary information for the trial at stage, which was generated by the SEQTEST procedure at the previous stage. As expected, the test statistic is in the continuation region. With ODS Graphics enabled, a boundary plot with the rejection and acceptance regions is displayed, as shown in Output 81.4.10.

At stage, the statistic is less than the upper boundary value, so the trial continues to the next stage. With the INFOADJ=PROP option (which is the default), the information levels at interim stages and are derived proportionally from the information levels in the BOUNDARY= data set. The information level at stage is computed as, where and are the information level and sample size at stage in the BOUNDARY= data set, and is the available sample size at stage. With the specified BOUNDARYKEY=BOTH option, the information levels and boundary values at future stages are modified to maintain both the and levels.
The "Design Information" table in Output 81.4.8 displays design specifications. The ODS OUTPUT statement with the TEST=TEST_PROP1 option creates an output data set named TEST_PROP1 which contains the updated boundary information for the test at stage. The BOUNDARYSCALE=MLE option displays the output boundaries in terms of the MLE scale. The BOUNDARYKEY=BOTH option maintains both the and levels. If the computed information level for stage is not the same as the value provided in the BOUNDARY= data set, the INFOADJ=PROP option (which is the default) proportionally adjusts the information levels at future interim stages from the levels provided in the BOUNDARY= data set. The DATA=DATA_PROP1 option specifies the input data set DATA_PROP1 that contains the test statistic and its associated sample size at stage, and the TESTVAR=PDIFF option identifies the test variable PDIFF in the data set.

The BOUNDARY= option specifies the input data set that provides the boundary information for the trial at stage, which was generated in the SEQDESIGN procedure.


 0 kommentar(er)
0 kommentar(er)
